

Pipeline
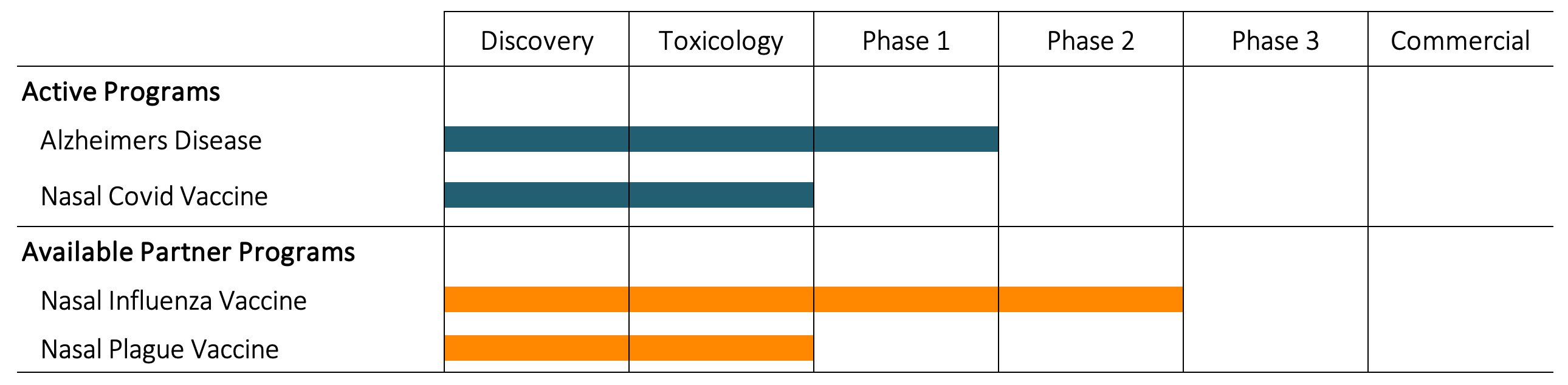
Alzheimers Disease
Inspirevax has collaborated with the laboratory of Dr. Howard Weiner at Brighams and Womens Hospital/Harvard Medical School since 2015 on the development of Protollin (BDX100) as an immunotherapeutic treatment for Alzheimer's Disease. Protollin, which was developed by ID Biomedical as a potent nasal vaccine adjuvant was shown to stimulate the reduction of beta-amyloid placque via and innate immune induction in mouse models of the disease. BWH and Inspirevax who co-own a patent for this indication have partnered with IMab Biopharma and Jiangsu Nhwa Pharmaceutical Co., Ltd. for the clinical development stage through a licensing agreement.
A phase 1 ascending dose tolerance clinical study was started in December, 2021.
Nasal Covid Vaccine
Inspirevax has pursued the development of a Nasal Covid Vaccine using a Proteosome adjuvant since early 2020. Since early 2021 we have teamed up with Oragenics Inc. on the development of a Nasal Covid Vaccine comprised of the BDX301 adjuvant and a trimeric recombinant spike protein antigen. This vaccine has given excellent preclincal immunogenicity and efficacy data with the observation of complete clearance of the SARS-CoV2 virus in vaccinated golden hamsters. Inspirevax has licensed BDX301 to Oragenics for the development of coronavirus vaccines. GLP toxicology studies are ongoing with an anticipated Phase 1 study in Q4 2022.
We believe the Nasal Covid Vaccine will offer distinct advantages including the induction of mucosal immunity, portability, safety and efficacy.
Nasal Influenza Vaccine
A Nasal Influenza Vaccine was developed by ID Biomedical using BDX200 or BDX300 adjuvants. 10 clinical trials were conducted in people aged 2-45. In general, the product was found to have a good safety profile and an efficacy profile similar to intramuscular influenza vaccines. The program was in 2008 in favor of other influenza projects including pandemic vaccine development. Inspirevax has acquired the rights to this indication through an exclusive License Agreement but has not reinitiated work on this indication. The remains available for sub-licensing.
Nasal Plague Vaccine
A Nasal Plague Vaccine was developed by ID Biomedical using the Protollin (BDX100) adjuvant. Preclincal studies demonstrated the induction of high quantities of both mucosal (IgA) and systemic (IgG) antibodies, protection against challenge and a good safety profile in GLP toxicology studies. Inspirevax has acquired the rights to this indication through an exclusive License Agreement but has not reinitiated work on this indication. The remains available for sub-licensing.